|
|
|
Wellmune Research - Allergies Last Updated: 02/23/2023 |
 Wellmune® Provided Significant Relief to Ragweed Allergy Sufferers A placebo-controlled, double-blinded study found that Wellmune® reduced allergy symptoms and improved the quality of life of individuals who suffer from ragweed allergy. Ragweed is a leading cause of seasonal allergy symptoms and affects 36 million Americans. Typical symptoms include nasal congestion, sneezing, itchy eyes and difficulty breathing. The cause is an immune system overreaction to ragweed pollen. Study Design The study equally divided 48 healthy subjects (31 female, 17 male; 39 ± 13 years of age) into two groups. One group consumed a placebo while the other a 250 mg serving of Wellmune® daily for four weeks during September/ October 2010 in an area of southeast Ohio where local pollen counts were high. Allergy surveys, including the validated Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), were used to assess differences in allergy symptoms. Study Results Individuals consuming Wellmune® experienced statistically significant (p<0.05) relief by several measures:
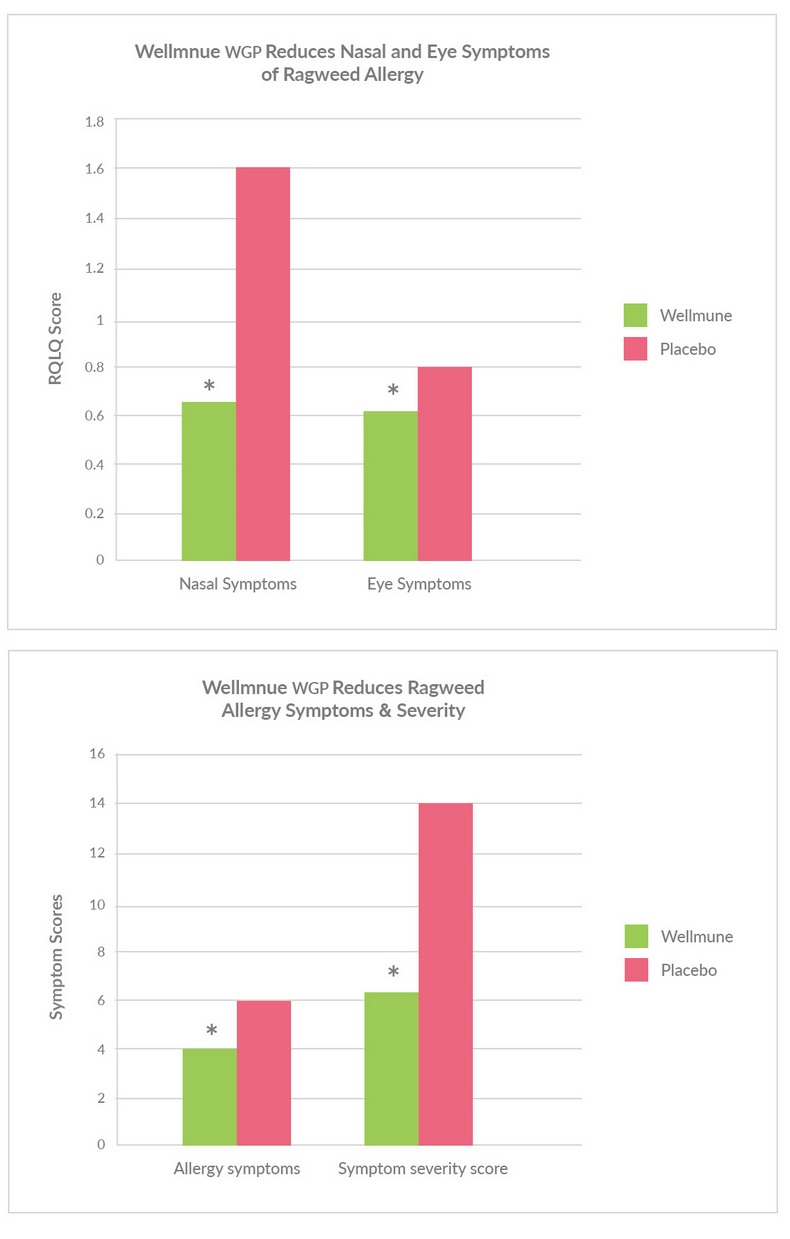 “ß-Glucan supplementation, allergy symptoms, and quality of life in self-described ragweed allergy sufferers.” Food Science & Nutrition. doi: 10.1002/fsn3.11 Tags:
Wellmune, Immune Health Basics, Kerry International, Beta Glucan, Beta 1, 3D Glucan, Wellmune Beta Glucan, Glucan 300, Immune System, Immune, Immunity, Allergies
|